Valence shell electron pair repulsion (VSEPR) model:
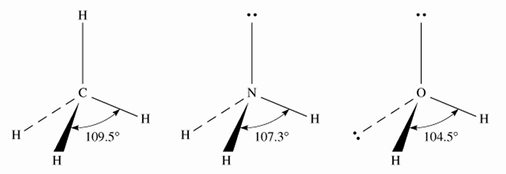
We use this to predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and non-bonding) pairs. The table below has molecules that have no non-bonding pairs:In all of these examples, there are no lone pairs. But it turns out that they can completely change the shape if present. The repulsion between non-bonding electron pairs (lone pairs) is greater than the repulsion between a non-bonding electron pair and a bonding electron pair which is greater than the repulsion of two bonding electron pairs.
(Also, this is all covered in Table 10.2 in the textbook. We're responsible for knowing this information.)
Predicting Molecular Geometry
1.) Draw Lewis structure for molecule2.) Count number of lone pairs on the central atom and number of atoms bonded to the central atom
3.) Use VSEPR to predict the geometry of the molecule
And that's it! Wear those flashcards out.


No comments:
Post a Comment